
Viable cells and pH
Figure 1a,b show the growth trend of bacteria and pH changes in FBRJ. Initially, the bacteria were inoculated with 5.5 log CFU/mL in BRJ. On the second day of fermentation, the fermentation process was performed slowly and steadily. But, on the first day of fermentation, bacterial production was also much higher than the second day of fermentation. For L. gasseri its value reached 10 log CFU/mL and for L. casei it was 8.8 log CFU/mL at the end of 48 h of fermentation. The pH was also four at the beginning of fermentation and during the fermentation of BRJ containing both LABs, pH decreased to 2.7 for L. gasseri and 2.9 for L. casei. In 2021, Li et al. reported that different strains of L. fermentum were inoculated with 7.5 log CFU/mL in blueberry juice24. By the end of 48 h of fermentation, the number of LAB in all subspecies had increased by more than 35% and some bacteria had reached more than 10 log CFU/mL24. In this study, a more than 81% increase in bacterial population was observed for L. gasseri and a 60% increase was observed for L. casei.
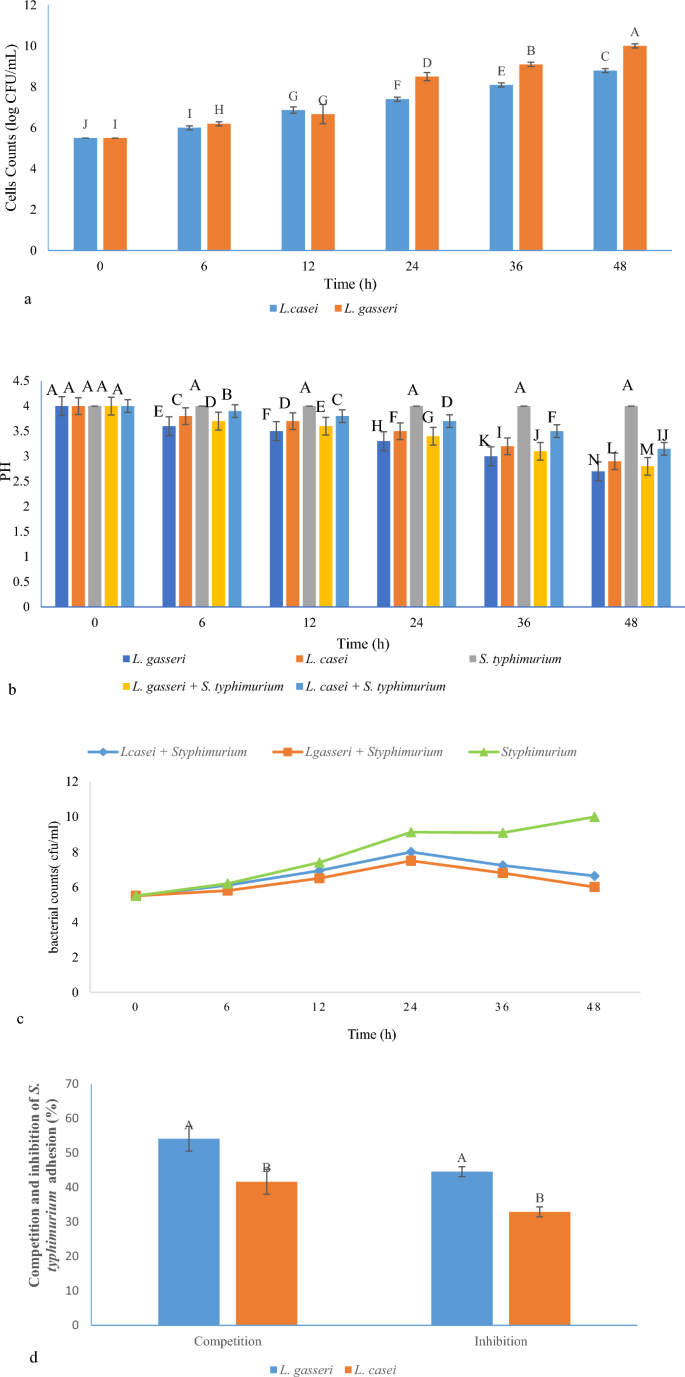
(a) Changes in cell counts of Lactobacillus strains in FBRJ; (b) changes in pH of BRJ during fermentation for 48 h; (c) changes in cell counts of S. typhimurium in various BRJ samples; (d) anti-adhesion assay (inhibition and competition of S. typhimurium in the presence of L. gasseri and L. casei strains) (different capital letters show the significant difference (p ≤ 0.05).
Two strains of Lactobacillus were used in BRJ against S. typhimurium. As shown in Fig. 1b, L. gasseri was effective in reducing pH compared to other treatments that pH reached 3.3 after 24 h and at the end of the fermentation process was 2.7, while pH reduction in fruit juice containing S. typhimurium did not occur during the experiment. It is noteworthy that due to the activity of each lactic bacterium alone in BRJ, the pH rate was lower than when each lactic bacterium was used in combination with S. typhimurium. Of course, this indicates acid production by LAB.
As shown in Fig. 1c, the changes in S. typhimurium count along with any of the tested Lactobacillus are different compared to BRJ and S. typhimurium without LAB. During the fermentation period where the LAB and S. typhimurium are present, the number of pathogenic bacteria increases in the first 24 h of fermentation. But after 24 h, the number of S. typhimurium decreases until the end of the fermentation process. When only pathogenic bacteria without LAB were present in BRJ, until the end of 48 h of fermentation, the number of S. typhimurium continuously increased. The mechanisms of the antibacterial activity of Lactobacillus strains appear to be multifactorial25. Lactobacillus strains inhibit the growth of pathogenic bacteria and sometimes even kill them by lowering the pH by producing acetic and lactic acid26.
Anti-adhesion effects of L. gasseri and L. casei against S. typhimurium
Intestinal infections initiate with the adhesion of the pathogenic bacteria to the mucosal surface. This process occurs through distinguishing the specific receptors by bacteria. Probiotic strains can adhere to gastrointestinal tract (GIT) receptors and block them from pathogens to prevent adhesion27,28. Competition between probiotics and pathogens for the same receptors can be a reason for balancing the GIT microbiota and preventing the infection. In this study, in the competition method after the simultaneous addition of L. gasseri and S. typhimurium, the adhesion rate of S. typhimurium decreased. The same process happened to L. casei to a lesser extent. Antimicrobial compounds such as hydrogen peroxide, bacteriocins, organic acids, and polysaccharides can reduce the adhesion. Other reasons include competition for nutrition and receptors27.
As shown in Fig. 1d, inhibitory effects on S. typhimurium were 44.5 and 32.9% for L. gasseri and L. casei, respectively. Competition effects on S. typhimurium were 54.1 and 41.6% for L. gasseri and L. casei, respectively. Currently, many studies show that L. casei may improve inflammatory bowel disease (IBD) by regulating the balance of intestinal microbiota and the host’s immune response and several researchers also reported that L. casei remarkably impedes the adhesion and invasion of pathogenic bacteria in the gut of food animals29. In addition to L. casei’s anti-adhesion and anti-inflammatory properties, it is compatible with food environment and has shown remarkable anti-diabetic properties, for example, L. casei NRRL-B-1922 has the high ability to grow and adapt in a Punica granatum juice environment, and showed acceptable antidiabetic properties30. Adhesion can be associated with different forms, which include the adhesion of these bacteria together or the adhesion of bacteria to host cells. An effective method is to prevent the formation of biofilm and colonization and thus prevent the initiation of infection by bacteria. Probiotic bacteria can compete nutritionally with other pathogens through the good adhesion ability to host’s intestinal cells, and by bindings to the receptor, they take the chance of adhesion from the pathogens. Moreover, antimicrobial compounds such as hydrogen peroxide, which is an oxygen catabolite, and bacteriocins, which are composed of antimicrobial protein substances, as well as organic acids such as lactic acid and acetic acid, are all involved in the anti-adhesion potential31.
In one of the studies that related to the anti-adhesion properties of Lacticaseibacillus rhamnosus GG, KU200656 and Lactiplantibacillus plantarum KU200656, Staphylococcus aureus (S. aureus) ATCC 6538 were able to work on pathogens such as Staphylococcus aureus, Listeria monocytogenes, Escherichia coli (E. coli) and Salmonella typhimurium to have 25–78% anti-adhesion properties31.
Another study showed that the consumption of L. casei WLCA02 significantly reduced the amounts of Salmonella in the ileum, colon and cecum and also in the liver and spleen that are outside the intestine, and this reduction was also observed in the feces32.
ABTS assay
ABTS (2,2′-azino-bis(3-ethylbenzothiazoline-6-sulfonic acid)) is a free radical used to measure antioxidant power. Phenolic compounds in fruit or vegetable juices give hydrogen to a strong oxidizing agent such as potassium persulfate in this reaction33. As indicated in Fig. 2a, at the beginning of fermentation, the rate of absorption of free radicals was 31% for both LABs. With the progress of fermentation, the absorption of ABTS radical for L. gasseri and L. casei strains reached 58 and 56.5% on the first day and 74 and 72.5% on the second day of fermentation of BRJ, respectively. There was a significant difference between different fermentation hours (0, 6, 12, 24, 36, and 48) in terms of antioxidant power to remove free radicals at the 5% level, but in each time of fermentation, there was no significant difference between L. gasseri and L. casei at the α = 5% level.
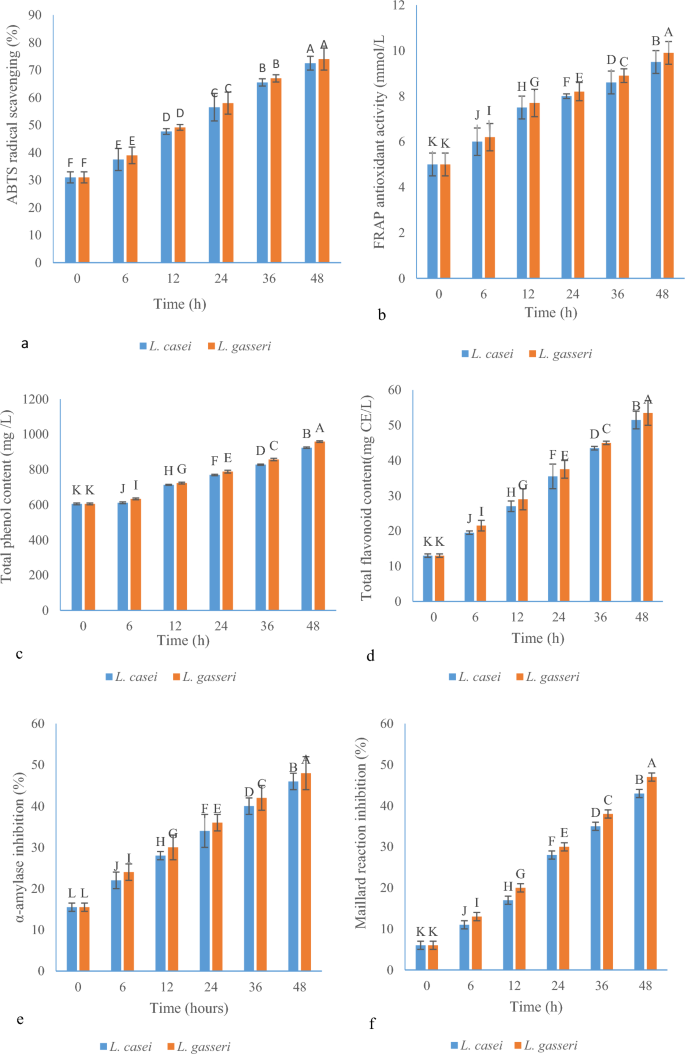
(a) ABTS scavenging for L. gasseri and L. casei; (b) antioxidant properties (FRAP) of BRJ fermented by Lactobacillus strains; (c) total phenolic content (mg/L) for L. gasseri and L. casei; (d) total flavonoid content (mg/L) of BRJ for L. gasseri and L. casei; (e) α-amylase inhibition of BRJ by L. gasseri and L. casei; (f) effect of Lactobacillus strains on Maillard reaction inhibition (%) during the fermentation of BRJ (different capital letter show the significant difference (p ≤ 0.05)).
In 2019, the improvement of the antioxidant power of apple juice by L. plantarum (ATCC 14917) was investigated and the increase of antioxidant power was reported by DPPH and ABTS methods at fermentation times of 24, 48 and 72 h. The reason for this increase in antioxidant power was found to be due to the increase in the amount of phenolic and flavonoid compounds in apple juice during fermentation34.
FRAP assay
The FRAP method is a process for measuring the antioxidant power based on the oxidation and reduction system. In this method, iron in the complex (FeIII-TPTZ) receives electrons in an acidic environment and as a result of this reaction, the color of the solution turns from yellow to blue35,36. The concentration of Fe2+ ions formed in the solution is found by measuring the absorbance at the wavelength of 593 nm. Higher absorption values in spectrophotometer indicate higher antioxidant power37. According to Fig. 2b, the result of the FRAP assay of BRJ at the end of 48 h of fermentation increased significantly compared to the beginning of fermentation. In this experiment, L. gasseri had higher antioxidant power than L. casei and at the end of 48 h FRAP antioxidant activities reached 9.9 and 9.5 mol/L for L. gasseri and L. casei, respectively. Similarly, in a recent study by Chen et al., L. acidophilus and L. plantarum improved the color and antioxidant activity of Strawberry during fermentation using the FRAP method38. At the end of 48 h of fermentation, it reached 14.54 and 14.52 mol/L for L. acidophilus and L. plantarum, respectively.
The antioxidant properties of LAB can be related to various factors, such as antioxidant enzymes resulting from their activity, exopolysaccharides and bioactive peptides produced by them, and finally manganese ions. In addition, beneficial bacteria in the intestine can produce bioactive food antioxidants using various enzymatic reactions39. In other researches, it is stated that LAB adapted to the environment of beverages such as jujube juice and Djulis can increase the absorption of free radicals, and thus the antioxidant properties improve through the increase of phenolic compounds40,41. In some studies, the effect of organic acids on antioxidant properties has been observed, such as lactic, caproic, acetic, lauric, and capric acid, which had increased significantly after the fermentation of jujube-wolfberry composite Juices and have increased the antioxidant properties42. Furthermore, phlorizin and caffeic acid increased antioxidant properties in apples after fermentation by LAB43. According to these studies, remarkable antioxidant properties were observed for LAB after the fermentation.
Total phenolic and total flavonoid content
In BRJ, the total phenolic and flavonoid content was 605 mg/L and 13 mg CE/L, respectively (Fig. 2c,d) and both LABs were able to significantly increase the total phenolic and total flavonoid content. For example, the amount of phenolic and flavonoid compounds reached from 788 to 958.33 mg/L and 37.5 to 53.5 mg CE/L after 24 and 48 h of fermentation for L. gasseri, respectively. As specified in the Fig. 2c,d, L. gasseri was stronger than L. casei. For L. casei, the amount of phenolic and flavonoid compounds reached 923.66 mg/L and 51.5 mg CE (Catechin)/L at the end of 48 h of fermentation. These results match the previous research. Previous studies have found that fermentation can increase compounds such as kaempferol and quercetin and other phenolic and flavonoid compounds44. Also, the previous studies indicated the effect of fermentation time on increased phenolic compounds in okra seed, which reached the highest level (1460 mg GAE/100 g) after 24 h of fermentation (1460 mg GAE/g), while at the beginning of fermentation, it was at its lowest (185 mg GAE/100 g)45.
There are different opinions about the increase in the amount of phenolic compounds due to the fermentation of LAB, but what is common among the theories is the activity of enzymes secreted by LAB. For example, in one study, Lactobacillus could produce a wider range of phenolic acids through ring fission, deglycosylation, reductive metabolism of phenyl acyl fragments, aromatic dehydroxylation and hydroxylation, decrease of double bonds (carbon–carbon) and all these operations were performed on anthocyanins38. In addition, in the fermentation process by Lactobacillus in Jussara pulp, secreted β-galactosidase and α-galactosidase enzymes were involved in the destruction and conversion of anthocyanins46.
In addition, in another research, it was reported that as a result of the activity of enzymes that are released in the fermentation process, compounds such as tannins, flavonoids, phenylpropanoid, and alkaloids are produced in a greater amount. In the fermentation process, LAB polymerizes complex phenolic compounds and converts them into simple phenolic compounds. It has been mentioned in a study that natural fermentation using microorganisms increases acidity and β-glucosidase produced by L. plantarum via the hydrolysis of complex phenolic compounds that enable them to produce simple phenolic compounds in high amounts47.
HPLC analysis
HPLC analysis of phenolic compounds of BRJ after 48 h of fermentation by L. gasseri is shown in Table 1. Among these 10 compounds, anthocyanins have a significant contribution in terms of quantity. Ellagic acid and cyanidin 3-glucoside had the highest amount among phenolic compounds and chlorogenic acid had the lowest amount. The order of phenolic compounds was as follows:
Ellagic acid > Cyanidin 3-glucoside > Gallic acid > Ferulic acid > Caffeic acid > Quercetin > Kaempfrol > p-Coumaric acid > Myricetin 3-glucoside > Chlorogenic acid.
Finally, the results of this study are consistent with the previous studies, as it has been reported that ellagic acid had the highest amount (2875 mg/kg) among other phenolic compounds of Raspberry leaf extract48.
α-Amylase inhibition
Compounds that inhibit the α-amylase enzyme prevent the increase of blood glucose and even reduce it after eating. In fact, they do this by slowing down the breakdown of carbohydrates in the small intestine and reducing postprandial hyperglycemia49. As seen in Fig. 2e, at the beginning of fermentation, the inhibition rate of the α-amylase enzyme was 15.5%, and with the start of the fermentation process by two LABs, the inhibition rate increased to a greater extent. At the end of 48 h of fermentation, the inhibition rate reached 48 and 46% for L. gasseri and L. casei bacteria, respectively. α-Amylase inhibitors prevent the absorption of starch from foods in the body, so-called a starch blocker. Because starch is a carbohydrate with a complex structure, it is not easily absorbed by the body unless it is broken down by α-amylase or other related enzymes50.
In previous studies, it has been stated that the inhibition of α-amylase by Morindalucida leaf extract may be due to the presence of several compounds present in the plant such as tannins, flavonoids and saponins49. In another study, α-amylase and α-glucosidase inhibitory activity of six groups of flavonoids were investigated and it was found that from the category of flavonols, myristin 64%, Quercetin 50%, Kaempferol 18% and Cyanidins from the category of anthocyanins inhibited α-amylase by more than 50%51. Therefore, due to the presence of the above compounds in the sample of FBRJ, the increase in inhibition of α-amylase can be related to the flavonols and anthocyanins of BRJ.
Maillard reaction
The Maillard reaction occurs between amino acids and reducing sugars, and brown aromatics compounds and UV-absorbing intermediates are the end products of this reaction52,53. LAB converts sugars into organic acids such as acetic, propionic, and lactic acids54 which leads to an increase in the ratio of non-reducing sugars to reducing sugars in the entire fruit juice environment, thus, the needed primary substance to carry out the Maillard reaction is removed, and the reaction is prevented55. Previous studies have also shown that fructose and glucose levels decreased during fermentation due to the activity of LAB and the consumption of simple sugars55,56. In the present work, L. gasseri and L. casei were able to prevent the Maillard reaction by 47 and 43%, respectively during 48 h of fermentation (Fig. 2f), while in the first of the fermentation, this amount was 6% in BRJ. Compared to the initial time of fermentation, the prevention of Maillard reaction was more effective in the first 24 h of the fermentation than in the second 24 h of the fermentation.
Antibacterial activity
In this study, three samples called fermented black raspberry juice (FBRJ), black raspberry juice (BRJ) and black raspberry Juice + Stevia extract (BRJ + SE) were used. All results were reported in terms of growth inhibition area (mm) in Table 2. All samples had antibacterial properties. E. coli were more sensitive than S. aureus to the samples and a larger inhibition zone was obtained (Table 2). The highest antibacterial power was related to BRJ + SE (25 g/L) against E. coli with diameters of 27 and 28 mm and the lowest was related to BRJ against S. aureus with a diameter of 6.7 and 7 mm, in VAT and HHP processing, respectively. The important point is that due to the fermentation process, the antibacterial activity of all fermented samples by L. gasseri and L. casei increased compared to unfermented samples. These results are consistent with the antibacterial properties of Fuji” apple juice. In that research, it was reported that samples fermented with L. acidophilus, L. casei and L. plantarum had higher antibacterial power than unfermented samples57.
Pasteurization processes and Stevia extract on the stability of phenolic and flavonoid compounds
Stevia extract was added at the rate of 12.5 and 25 g/L to the BRJ samples and pasteurization processes such as HHP and VAT were applied to the samples. The amount of phenolic and flavonoid compounds was measured on the 28th day and the amount of reduction of these compounds after 28 days was also calculated to determine the exact effect of extract and pasteurization processes on phenolic and flavonoid compounds. As shown in Fig. 3a,b, all fermented and unfermented raspberry samples mixed with Stevia extract at two levels of 12.5 and 25 g/L and subjected to different pasteurization processes have a significant difference from each other on day 28 in terms of the amount of phenolic compounds. The fermented and unfermented sample of BRJ that was mixed with Stevia extract at a level of 25 g/L and subjected to the HHP process (FBRJ + SE 25 (HHP)) and (BRJ + SE 25 (HHP)) was the highest in terms of the amount of phenolic compounds, and the fermented and unfermented sample of BRJ, which was mixed with Stevia extract at the level of 12.5 g/L and was subjected to VAT process (FBRJ + SE 12.5 (VAT)) and (BRJ + SE 12.5 (VAT)) were the lowest in terms of phenolic compounds. The order of the values of phenolic compounds is shown in Fig. 3a,b.
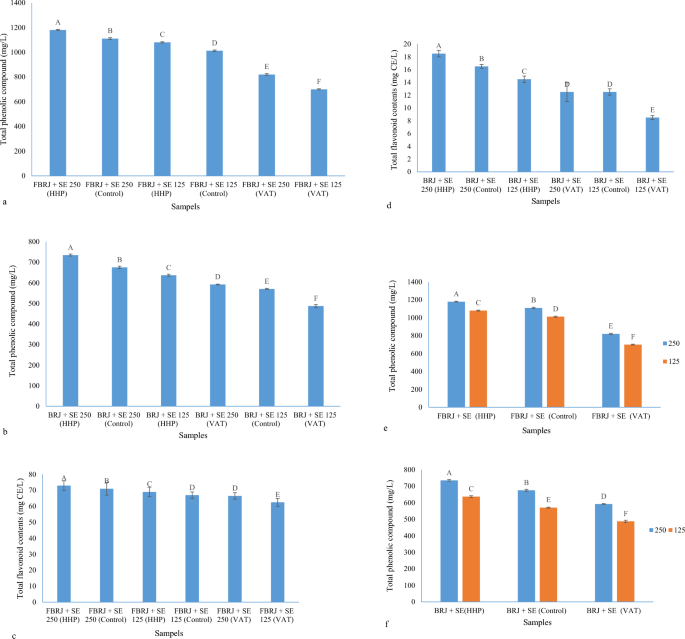
(a) Total phenolic compounds of FBRJ samples combined with Stevia extract on the 28th day; (b) total phenolic compounds of unfermented BRJ samples combined with Stevia extract on the 28th day; (c) total flavonoid contents of FBRJ samples combined with Stevia extract on the 28th day; (d) total flavonoid contents of unfermented BRJ samples combined with Stevia extract on the 28th day; (e) simultaneous effect of concentration and pasteurization process on phenolic compounds (FBRJ) after 28 days; (f) simultaneous effect of concentration and pasteurization process on phenolic compounds (unfermented BRJ) after 28 days (different capital letters show the significant difference (p ≤ 0.05)).
Regarding the remaining flavonoid compounds after 28 days, the results were according to phenolic compounds with slight differences. There was a significant difference between all samples, except FBRJ + SE 12.5 (Control) and FBRJ + SE 25(VAT) samples, as well as between BRJ + SE 25 (VAT) and BRJ + SE 12.5 (Control) samples that there was no significant difference between the mentioned samples. There was also no significant difference in terms of quantity and the last-mentioned samples were even completely equal in terms of quantity (Fig. 3c,d).
The order of strength of samples for flavonoid contents is as follows:
$${\text{FBRJ}} + {\text{SE}}\;25\;({\text{HHP}}) > {\text{FBRJ}} + {\text{SE}}\;25\;({\text{Control}}) > {\text{FBRJ}} + {\text{SE}}\;12.5\;({\text{HHP}}) > {\text{FBRJ}} + {\text{SE}}\;12.5\;({\text{Control}}) > {\text{FBRJ}} + {\text{SE}}\;25\;({\text{VAT}}) > {\text{FBRJ}} + {\text{SE}}\;12.5\;({\text{VAT)}}$$
The simultaneous effect of concentration and pasteurization process on phenolic compounds after 28 days can be seen in Fig. 3e,f, and the HHP process is the best process for pasteurization of fermented and unfermented samples of BRJ with Stevia extracts at different levels. The VAT pasteurization sample was so destructive that even the amount of phenolic compounds remained in this method was lower than the control sample, and these amount at the end of 28 days for the fermented samples that were combined with 25 and 12.5 g/L of Stevia were 820 and 700 mg/L, respectively and in the HHP process 1180, 1080 and 1110 and 1012 mg/L were reported for the control sample, which indicates the positive effect of the HHP process and the negative effect of the VAT process on the pasteurization of phenolic compounds. The same trend prevailed for non-fermented samples; the only difference was that the non-fermented samples that were combined with Stevia extract were at a lower level than the non-fermented samples in terms of the amount of remaining phenolic compounds. For BRJ + SE 25 (HHP) and BRJ + SE 12.5 (HHP) and BRJ + SE 25 (Control) and BRJ + SE 12.5 (Control) and BRJ + SE 25 (VAT) and BRJ + SE 12.5 (VAT), respectively, the values of 735, 675, 637, 592, 570, 487 mg/L were obtained and as it is known, the higher concentration of Stevia extract is more reliable in the stability of phenolic compounds than lower concentrations. The same effect was seen for flavonoid compounds. These results are consistent with the results of Rios-Corripio et al. They investigated the HHP process with different pressures and times for the stability of phenolic and flavonoid compounds and anthocyanins of fermented pomegranate juice. They compared the HHP process with VAT (63 °C/30 min) and HTST (72 °C/15 s) methods, as well as the control. HHP method, HTST, control and VAT were the best to worst pasteurization processes to preserve phenolic and flavonoid compounds and anthocyanins of fermented pomegranate juice, respectively58.
As shown in Fig. 4a–d, the amount of reduction of phenolic and flavonoid compounds for fermented and non-fermented samples of BRJ without adding Stevia extract is higher than in other samples. For example, for the sample fermented by L. gasseri and the non-fermented sample to which no extract was added, the reduction of phenolic compounds was observed by 223 and 260 mg/L, respectively, which was the largest reduction among the samples. For the fermented and non-fermented samples of BRJ to which 25 g/L of Stevia extract was added and subjected to the HHP process, 70 and 100 mg/L reduction of phenolic compounds was observed, respectively, which was the lowest decrease among the samples. Also, in the case of flavonoids, a decrease in flavonoid compounds was observed by 9.5 and 10.5 mg CE/L, respectively, for the sample fermented by L. gasseri and non-fermented to which no extract was added, which had the largest decrease among the samples. For the fermented and non-fermented samples of BRJ to which 25 g/L of Stevia extract was added and subjected to HHP process, 5.5 and 6.5 mg/L reduction of flavonoid compounds was observed, which was the lowest reduction among the samples. It can be concluded that increasing the concentration of Stevia extract along with the HHP process can help the stability of phenolic and flavonoid compounds after 28 days of storage in the refrigerator. In a study, the stability of phenolic, antioxidant, and antidiabetic compounds of roselle drink was investigated using Stevia and citric acid; It was reported that the addition of Stevia to the beverage increased the stability of gallic acid, rosmarinic acid, epigallocatechin gallate, and quercetin during storage for 12 days. It was also reported about anthocyanins that the content of total anthocyanin after storage had a slight increase in the content of pelargonidin-3-glucoside, cyanidin-3-glucoside, and peonidin-3-glucoside after storage and these compounds are pigments that have an important effect in increasing the antioxidant property in fruit juice and over time due to the changes that occur in the B-ring of anthocyanins compounds, 3,4-dihydroxybenzoic and phydroxybenzoic, 3,4,5-trihydroxybenzoic acids are produced and one of the characteristics of the produced compounds is that they are hydrogen donors and inhibit the activity of free radicals and thus increase the antioxidant activity. In addition, they increase the amount of total phenolic compounds during storage59. According to the above, for this research, such a result can be taken in relation to the changes in anthocyanins and the increase of phenolic acids in BRJ.
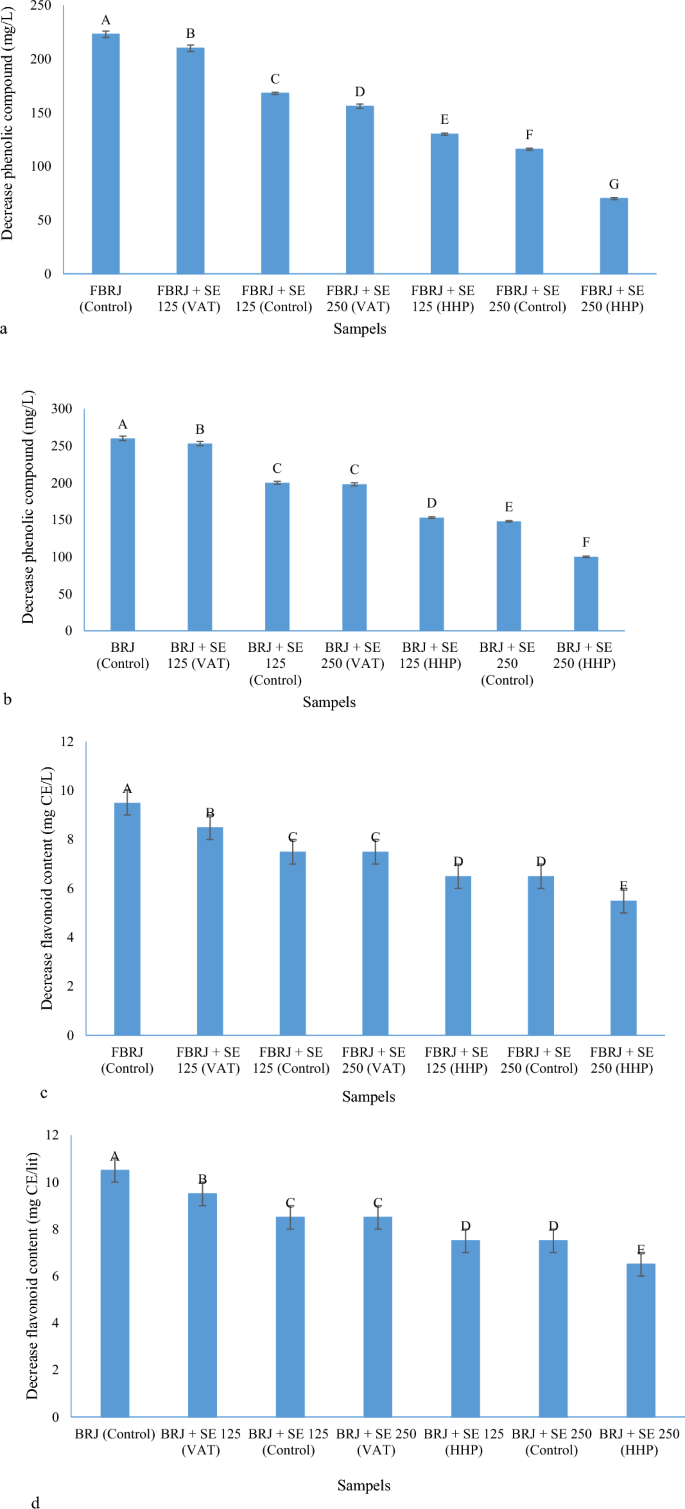
(a) Decrease phenolic compound for FBRJ samples during 28 days; (b) decrease phenolic compound for unfermented BRJ samples during 28 days; (c) decrease flavonoid content for FBRJ samples during 28 days; (d) decrease flavonoid content for BRJ samples during 28 days (different capital letters show the significant difference (p ≤ 0.05)).
In addition, in previous research conducted on Stevia, it has been determined that this plant has many glycosides and is mainly composed of steviol, which can link with the surrounding sugars or glycosides with these glycosidic bonds60. Therefore, it can have a protective role on the phenolic compounds of BRJ over time. Another study showed that glycosides and pigments in Stevia have been preserved to a significant extent even after bread processing and have preserved the antioxidant property of bread61; therefore, Stevia has many pigments that can bond with the pigments of BRJ and create a protective complex.
It has been reported that phenolic compounds extracted from plants have medicinal properties, including anti-diabetic properties and anti-inflammatory properties62,63. It has also been reported that the extraction of phenolic compounds in three forms, free, esterified and insoluble-bound, from (Cucumaria frondosa) tentacles significantly control the amount of secondary oxidation products in fish64. On the other hand, raspberries have valuable terpenes such as α-pinene, β-myrcene and linalool, and among these compounds65, it has been reported that α-pinene, in addition to having therapeutic properties such as protecting the heart and being anti-cancer, also has flavoring properties, so the extract and essential oil of this fruit together with Stevia can be used as a natural preservative in food for a long time66.



